What Best Describes the First Law of Thermodynamics
The first law of thermodynamics is about the law of conservation of energy for a thermodynamic system. What is the First Law of Thermodynamics.

Solved Which Of The Following Statements Best Describes The Chegg Com
Which of the following statements best describes the first law of thermodynamics.
. 500 104 J of heat when 200 104 J of heat is exhausted. The first law describes how thermal energy is conserved but not the direction it moves. The first law of thermodynamics is given as ΔU Q W where ΔU is the change in internal energy of a system Q is the net heat transfer the sum of all heat transfer into and out of the system and W is the net work done the sum of all work done on or by the system.
300 104 J. Here ΔU is the change in internal energy U of the system. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system plus the net work done.
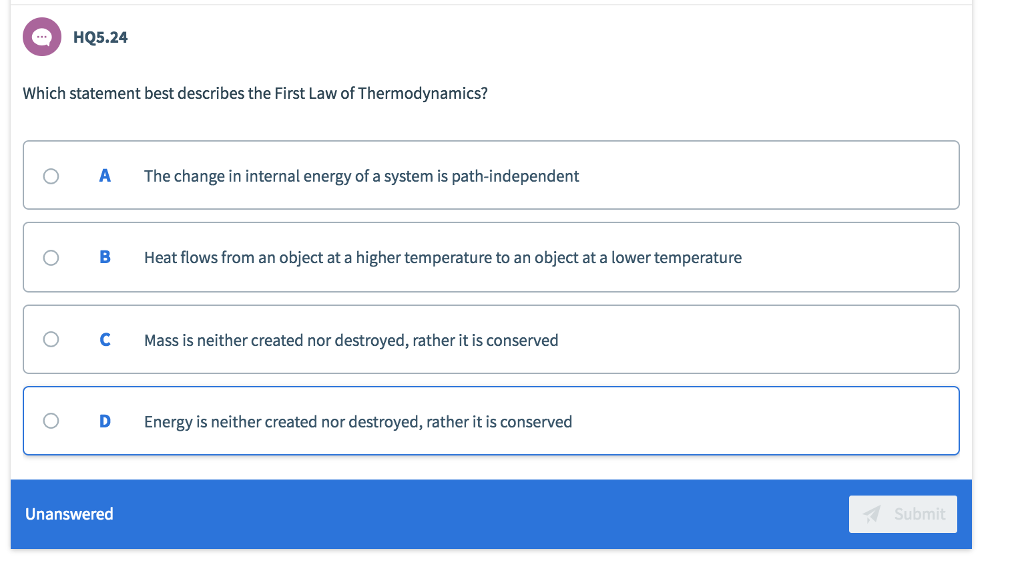
Energy is neither created nor destroyed rather it is conserved Explanation. The First Law of Thermodynamics. The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system.
In equation form the first law of thermodynamics is ΔU QW Δ U Q W. The local power company burns fossil fuels which pollute the environment to generate electricity. Between two systems the change in the internal energy is equal to the difference of the heat transfer into the system and the work done by the system.
Which one of the following describes the first law of thermodynamics. Evaluate the statements and choose the statement that best describes how the windmill technology benefits the environment. A thermodynamic system in an equilibrium state possesses a state variable known as the internal energyE.
It deals with the total amount of energy in the universe and in particular it states that this total amount does not change. It states that the energy can neither c. The change in thermal energy of a system is equal to the energy transferred into.
Which of the following. This first law could be considered bookkeeping. The First Law of Thermodynamics is another name for the Law of Conservation of Energy.
The first law of thermodynamics states that energy is neither created nor destroyed. Include differential and integral calculus and courses more advanced than first-year physics and chemistry in five of the following seven areas of engineering science or physics. Which of the following statements best describes a primary mechanism by which the energy released in ATP hydrolysis is used directly to drive endergonic chemical reactions in a cell.
Put another way the First Law of Thermodynamics states. Energy can be created from matter or used to produce matter. Metabolism uses all of an organisms resources.
The first law of thermodynamics is a version of the law of conservation of energy specialized for thermodynamical systems. Which of the following statements best describes the first law of thermodynamics. ΔU Q W where ΔU is the change in the internal energy Q is the heat added to the system and W is the work done.
Energy always changes from a more useful more concentrated form to a less useful less concentrated form in a closed system of constant mass the energy involved in any physical or chemical change is neither created nor destroyed ut merely changed from one form to another. The first law of thermodynamics can be captured by the following equation. Which of the following statements correctly describes a heat engine.
In equation form the first law of thermodynamics is ΔU Q W. Heat transfer into them takes place so that they can do work. Which best describes the second law of thermodynamics.
Energy cannot be created or destroyed. It is used extensively in the discussion of heat engines. 2 points all vibrating bodies produce sounds are we.
Photosynthesis converts light energy a form of kinetic energy into chemical energy. The first law of thermodynamics is related to the conservation of which quantity. Following devices does not convert heat energy to mechanical energy.
Heat engines are a good example of the application of the 1st law. Statics dynamics Strength of materials stress-strain relationships Fluid mechanics hydraulics Thermodynamics Electrical fields and circuits. The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes.
As per the question the first law discusses that heat is a kind or form of energy and. Ack to the power company. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system.
Metabolism manages the increase of entropy in an organism. Some useful energy is lost as heat whenever an energy transfer occurs. Which is the best description of the first law of thermodynamics.
Thermodynamics is demonstrated as the branch of physics that deals with the associations or relationships between heat and other energy forms like mechanical chemical and electrical. Windmills do not pollute the environment. Which of the following best describes the first law of thermodynamics.
Energy transfers are always 100 efficient. It states that the energy used and released in any reaction must be balanced. The first law makes use of the key concepts of internal energy heat and system work.
The first law of thermodynamics thinks big. Metabolism depends on a constant supply of energy from food. First Law of Thermodynamics for a Closed System.
Metabolism consists of all the energy transformation reactions in an organism. Energy can be transformed in different.

Solved Hq5 24 Which Statement Best Describes The First Law Chegg Com
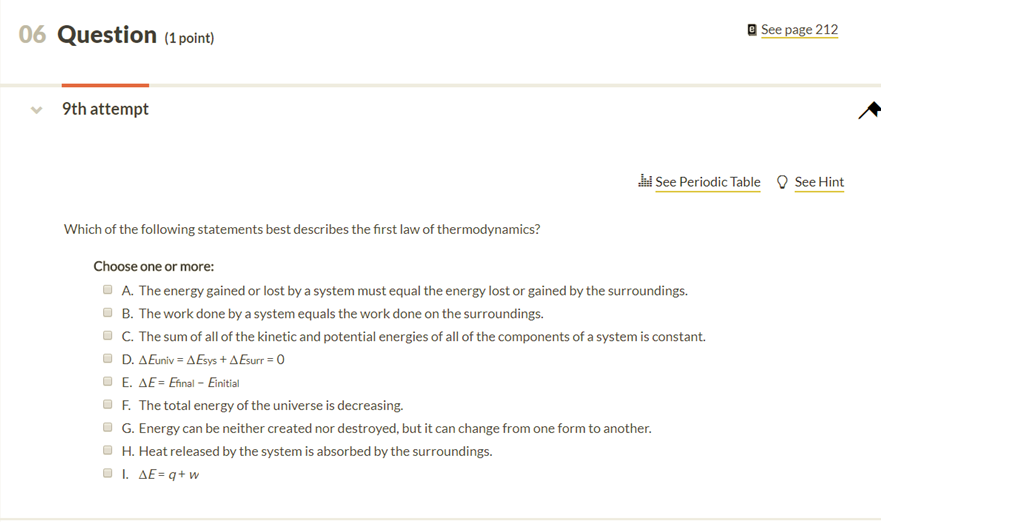
Solved Which Of The Following Statements Best Describes The Chegg Com
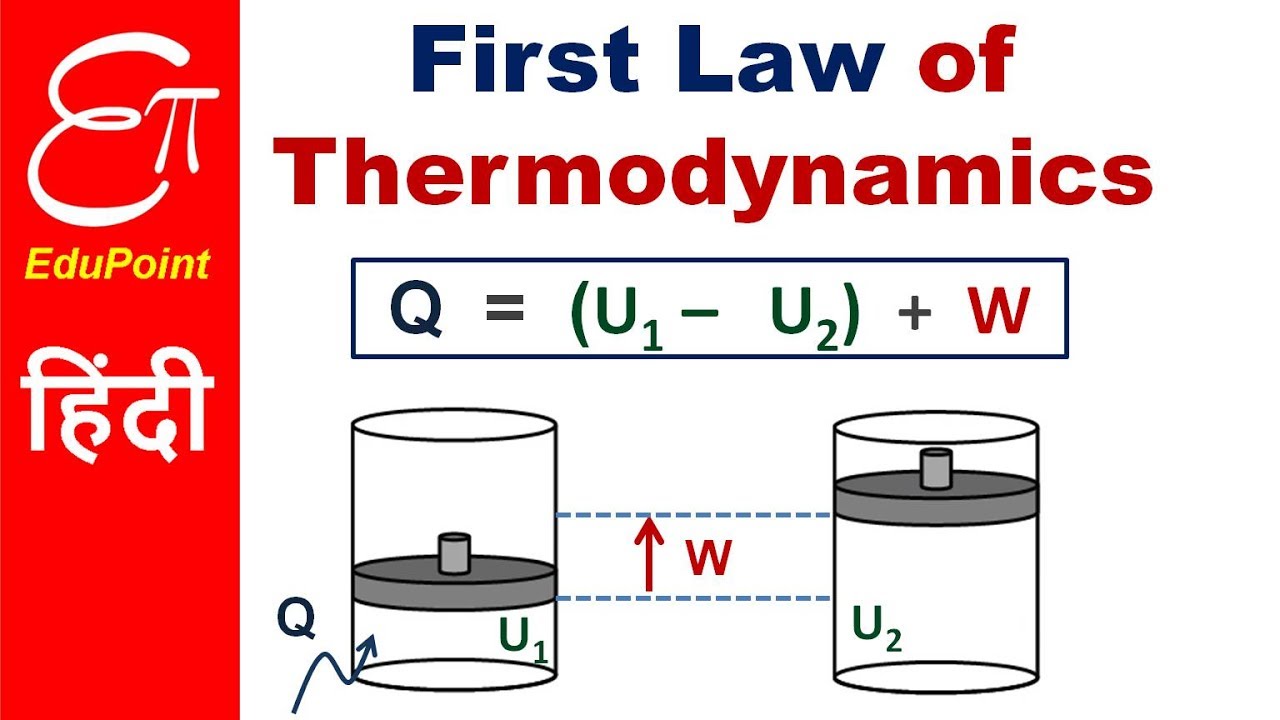
The First Law Of Thermodynamics Lecture Explained In Hindi Youtube

What Is The First Law Of Thermodynamics Article Khan Academy
0 Response to "What Best Describes the First Law of Thermodynamics"
Post a Comment